Dr. Lindsay Williams received her Doctorate of Dental Surgery at the University of Maryland. She began her journey practicing general dentistry for eight years and eventually started treating patients for sleep and airway. After realizing that this was her passion she decided to hang up her drill and has been dedicated to solely treating patients for sleep and airway ever since. Dr. Williams works at the Vivos Center in Highlands Ranch, Co, treating both adults and children in addition to instructing courses at the Vivos Institute in Denver.
Tip Tuesday: Vivos Therapeutics Receives FDA 510(K) Clearance

It’s an exciting time at Vivos: “For the first time, the FDA has formally recognized the benefits of our proprietary core technology in our DNA appliance (without mandibular advancement) as an effective treatment for mild-to-moderate OSA in adults.”

Vivos Therapeutics Receives FDA 510(k) Clearance of its Flagship DNA Oral Appliance for Treatment of Obstructive Sleep Apnea
| Source: Vivos Therapeutics, Inc
LITTLETON, Colo., Jan. 04, 2023 (GLOBE NEWSWIRE) — Vivos Therapeutics, Inc. (“Vivos” or the “Company”) (NASDAQ: VVOS), a medical technology company focused on developing innovative treatments for patients suffering from dentofacial abnormalities and/or mild-to-moderate obstructive sleep apnea (OSA) and snoring in adults, today announced a brand new clearance from the U.S. Food and Drug Administration (FDA) for its proprietary DNA appliance (daytime-nighttime appliance).
The FDA 510(k) clearance for the DNA appliance as a Class II device gives rise to a completely new treatment regimen for mild-to-moderate OSA. Making this development even more meaningful is that the DNA device for palatal expansion is Vivos’ longest standing appliance with the widest use among Vivos-trained dentists, which Vivos anticipates will now increase the ease of adoption of the DNA for treating OSA.
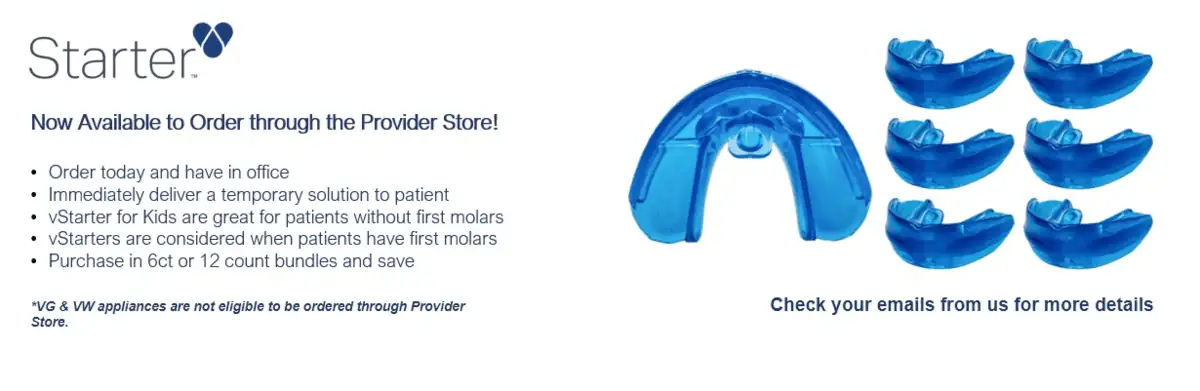
EMA-NOW is a temporary, 30-day, semi-custom, medical oral appliance.
EMA-NOW Temporary Sleep Appliance – 11ct. Bundle
$999.00
EMA-NOW Temporary Sleep Appliance
$99.00
If you haven’t been to The Vivos Institute yet, what’s stopping you from coming? Attend one of our upcoming in-person courses!
Block 1 and Block 2 Live Practical Training
Our live training courses are available to all Vivos VIPs who have completed the Online Block Training. This is a hands-on course where you will review everything learned from online block training, go through the record process as a patient, and take the records as the doctor. You will also get hands-on training with Vivos guides and the DNA, mRNA, and mmRNA appliances.
*You must have completed Online Block Training and attended Live Block 1 practical training to attend Block 2 live.
See what our VIPs and their amazing team members are saying about The Vivos Institute!

Join us for a NEW live webinar next week!
January 13 | 11AM MDT

Why Attend?
11 AM MDT / 12 PM CDT
This course will provide insight into understanding the design, function, and use of the Vivos Guide eruption appliance series in pediatric patients. In this course, you will:
- Understand the benefits of early childhood development, early intervention,
- Understand the basic concepts of the importance of the tongue and nasal breathing
- Understand the basic design and features of the Vivos Guide series
- Understand the basic use, indications, and contraindications of Vivos Guide appliances
Meet the Speaker



Join Dr. Toshi Hart & Dr. Martin Gorman’s POD Call
Pearls & Buteyko Breathing
Hello! This is a friendly reminder to join Dr. Harts POD.
She will be reviewing clinical pearls and basic buteyko breathing techiques
Thursday, January 12th 4:30 PM PST